About Us
Arbele’s mission is to create more effective treatments for GI cancers
We are a clinical stage biotechnology company with our core proprietary technology platform centered on immunotherapy – TriAx Multispecific biologics as well as artificial-immunosurveillance AI-CAR technology for the treatment of gastrointestinal cancers.
Our main focus is on our proprietary biomarker, Cadherin-17 (CDH17), a highly specific target for GI cancers.
Our Focus
Gastrointestinal Cancers
Gastrointestinal cancers presents a pressing challenge marked by unmet medical needs. Stomach, pancreas, colon, bile duct, and liver cancers collectively bear a significant burden, characterized by their high mortality rates and limited treatment alternatives. The complexity of these malignancies demands a new era of innovative therapeutic interventions that can effectively target the intricate molecular pathways driving tumor growth and progression.
Arbele is steadfast in its mission to bridge this therapeutic gap, pioneering groundbreaking drug development to provide hope and healing to patients grappling with these diseases. Our commitment to advancing scientific understanding and delivering novel solutions underscores our dedication to transforming the landscape of gastrointestinal cancer treatment.

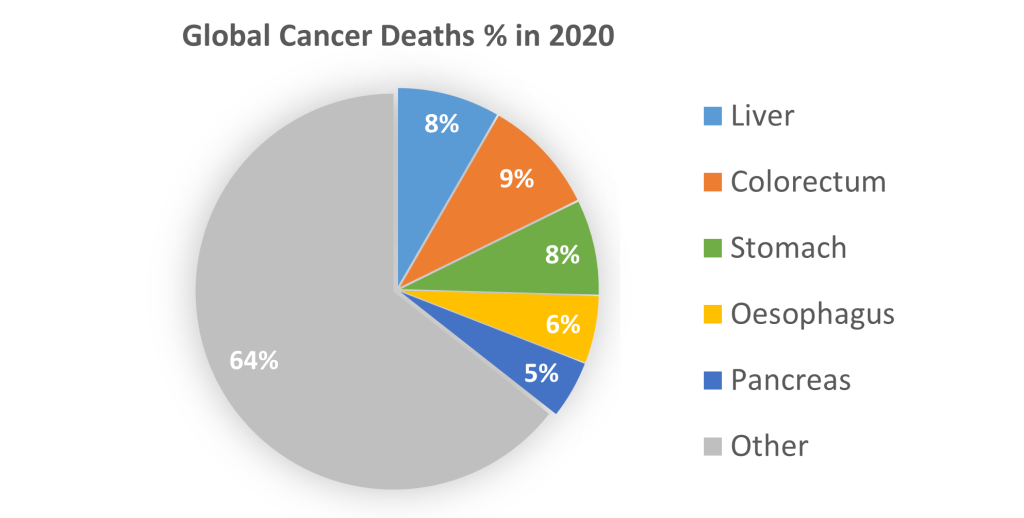
Data from GLOBOCAN 2020; International Agency for Research on Cancer, WHO
